Recently, we learned a lot about the Periodic Table of Elements. The Periodic Table has all the known atoms that the world is made up of. Each atom has electrons, which have a negative charge, neutrons, which have a neutral charge, and protons, which have a positive charge. To learn more about atoms, go see one of my previous posts.
Anyway, the Periodic Table has different columns, which are called groups. The elements in each group have the same number of electrons in the outer orbital. Some names of groups are noble gasses or halogens.
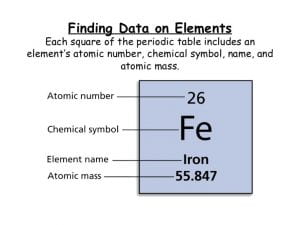
If you pull up a chart with data on a certain atom, you’ll see that this chart has some numbers on it. These numbers are called the atomic mass of an atom and the atomic number. The atomic mass is the number of protons and neutrons in an atom. The atomic number is the number of protons in an atom.
There are two types of atoms; A neutral atom and an ion. A neutral atom is where the number of protons in an atom equal the number of electrons. An ion is when the number of protons does not equal the number of electrons in an atom. If there are more protons in an atom, that atom has a positive charge. If there are more electrons, the atom has a negative charge.
Below, I posted a picture of an atom that is labeled.