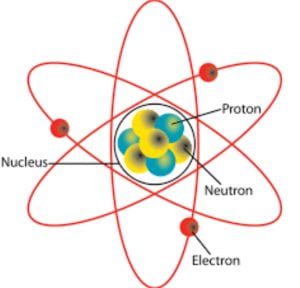
 Atoms consist of the nucleus and the electrons. The nucleus is both the protons and neutrons. The nucleus is surrounded by the electrons. The nucleus is incredibly small. For instance, if the atom was the size of a full-sized football field, the nucleus would be the size of a marble.
Atoms consist of the nucleus and the electrons. The nucleus is both the protons and neutrons. The nucleus is surrounded by the electrons. The nucleus is incredibly small. For instance, if the atom was the size of a full-sized football field, the nucleus would be the size of a marble.
You start with a ball the size of a grapefruit. This grapefruit is filled with atoms. In order to see the atoms within it, you must make the grapefruit the size of the Earth. Then, you can see the whole world filled with the atoms within the grapefruit. Each atom is the size of a blueberry. Although, you still can’t see the nucleus, so you enlarge one blueberry to the size of a full-sized football field. Then, the nucleus is the size of a marble.
Electrons are negative, protons are positive, and neutrons are neutral. The neutrons create a buffer that allows the electrons and neutrons to coexist. The neutrons allows the protons to exist. Without protons, atoms couldn’t exist. Without atoms, nothing could exist.